


The Implantable Miniature Telescope

Clinical Trial Begins of the IMPLANTABLE MINIATURE TELESCOPE for PATIENTS with advanced MACULAR DEGENERATION
Tucson, Arizona USA- Robert M. Kershner, MD, FACS of the Eye Laser Center in Tucson, Arizona
announced today that they are currently enrolling eligible patients with macular
degeneration to participate in a nationwide clinical trial of the Implantable
Miniature Telescope (IMT™). The multi-center trial
will examine if the IMT, an investigational device implanted inside the eye, can
safely improve visual acuity in patients with age-related macular degeneration
(AMD), the leading cause of blindness and visual impairment in the western
world.
“Patients
who have lost functional vision as a result of advanced Age-Related Macular
Degeneration have not had many options for improved vision,” said
Doctor Robert M. Kershner, MD, FACS of the Eye Laser Center
in Tucson. Doctor Kershner is
the local principal investigator for this study.
"This amazing device offers the first hope of restoring visual function to
people who are severely affected by this disease."
This
Phase II/III investigational trial will include approximately 200 patients from
30 leading research universities and ophthalmology centers across the U.S of
which the Eye Laser Center in Tucson is one.
The study is intended to assess the effectiveness of the IMT to safely
improve vision in patients with advanced AMD or Stargardt’s macular dystrophy,
an inherited disease of the macula. Patients
who have either of these conditions and wish to know if they are eligible to
participate in the trial can contact the Eye Laser Center in Tucson by calling
520-797-2020, faxing their information to 520-797-2235 or by e-mailing to info@asiteforeyes.com.
Eligible
patients must be over the age of 55 and have advanced dry AMD, disciform scar
(wet form of AMD), or Stargardt’s macular dystrophy in both eyes.
Visual acuity cannot be better than 20/80 or worse than 20/630 in both
eyes. No other major eye disease
can be present, except for cataract. Individuals
who have undergone surgery for cataracts in both eyes are not eligible
for this clinical trial.
Candidates
must be able to attend all follow-up visits during this 2-year study.
They must also attend scheduled vision training appointments after the
implantation procedure to help adapt to the implantation of the device.
Enrolled patients will receive the device and
study related procedures at no cost.
AMD
is the leading cause of vision loss in patients over the age of 50.
AMD is characterized by degenerative changes to the macula, the most
sensitive part of the retina in the back of the eye. The retina contains light sensing cells that capture visual
information that is sent to the brain. The
macula is responsible for detailed central vision that is required for reading,
shopping, and other daily activities.
AMD
is generally classified into its dry (atrophic) and wet (exudative) forms.
The dry form of the disease is more common and progresses slowly, while
the less common wet form can cause rapid vision loss. Late-stage dry AMD and wet
AMD that has scarred, or disciform AMD, are advanced forms of the disease that
can cause moderate to severe vision loss.
Nearly
15 million Americans have some form of AMD, according to the National Institutes
of Health’s National Eye Institute, and over 1.6 million older Americans have
the advanced form of the disease. To date, there is no accepted treatment for
dry AMD and existing treatments for wet AMD have variable results. The IMT
clinical trial focuses on the advanced form of the disease that robs central
vision from those patients it affects.
About the IMT
 |
|
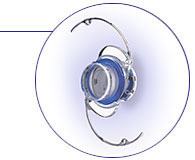
The
IMT is a micro-sized precision telescope approximately the size of a pea. It is designed to magnify images onto the retina.
By magnifying images for patients with macular degeneration, it is hoped
the blind spot caused by the disease is reduced, allowing for improved central
vision and associated function in daily activities such as recognizing people,
watching television, and reading the newspaper.
The IMT is implanted inside one eye by an ophthalmologist in an
outpatient procedure much like cataract surgery.
Placement inside the eye allows the advantage of utilizing natural eye
movements to scan the environment and reading materials, as well as providing a
natural cosmetic appearance. The
non-implanted eye will continue to provide peripheral or “side” vision for
the patient.
For
more information on the IMT and the clinical trial, please visit the study
sponsor’s company web site at ,
call them at
1-888-528-0006
or contact us through this website at: info@asiteforeyes.com.
For
more information about the Eye Laser Center and other pioneering work of Robert
M. Kershner, MD, FACS or for patient inquiries visit:
or call 520-797-2020, fax 520-797-2235
Robert M. Kershner, MD, FACS is an internationally recognized ophthalmic surgeon. He is a frequent contributor to health columns, has written over two hundred scientific articles, contributed to sixteen textbooks and lectures on eye microsurgical techniques to surgeons the world over. He specializes in cataract and refractive surgery at the Eye Laser Center in Tucson, Arizona. Copies of his articles and chapters of his book "Lessons from the Practice-The Gift of Sight" are available for free download from his website or call (520)797-2020 for a consultation.